We are back and talking about another anti-obesity medication that is technically a combination of 2 older medications that have been around for a while; kinda like Contrave!
Today’s medication goes under the brand name Qsymia, or Phentermine and Topiramate. If you live outside of the US of A, you are likely not very familiar with Qysmia specifically. That is because the US is one of the few countries where this medication is currently approved.
I know it is not available here in Canada, and from what I can tell, a number of European countries have also not approved its sale. That is likely because of the tumultuous history the Phentermine component of Qsymia has had! As well, since I am in Canada, I have limited experience with it!
Perhaps you have heard of the Fen-Phen debacle?
Fen-Phen what?
 Phentermine originally came to market in 1959 and is currently the longest-standing weight-management medication in the world. Unfortunately, when it was combined with fenfluramine to make Fen-Phen in the 1990s it got a bit of a black mark on its record.
Phentermine originally came to market in 1959 and is currently the longest-standing weight-management medication in the world. Unfortunately, when it was combined with fenfluramine to make Fen-Phen in the 1990s it got a bit of a black mark on its record.
Fen-Phen was helping people with weight-loss but it was also found to be causing issues with heart valves. While I am no cardiologist, I am pretty sure valves are important for proper heart function and living. Fortunately, it was found that only fenfluramine was the bad guy, not Phentermine. As such, fenfluramine was pulled from the market and Phentermine was allowed to continue its reign as the longest-standing weight-loss medication on the market.
Topiramate on the other hand was originally brought to market as an anti-seizure medication and has since been shown to be beneficial for migraine prophylaxis as well as other off-label uses.
Topiramate was also considered a stand-alone medication for obesity management as people who took it for other indications noted a decrease in appetite and weight-loss. However, it never made it as a stand-alone agent for obesity as it caused some issues with mood and memory. It actually has a history of being called the California drug as it can make you skinny and stupid… As a side note, for the more easily triggered individuals, I am not saying people from California are stupid. I am just telling you the history of the drug. So don’t @ me in the comments. Kapeesh?
Anyways in the case of Phentermine and Topiramate, it was known that both can lead to and support weight-loss. However, either their effectiveness or their side effects at higher doses were not ideal. So what better opportunity to then combine both medications and use each one at lower doses to not only mitigate dose-dependent side effects but also hopefully increase effectiveness.
Thus Qsymia was born!
And how effective is the combination? Aka Qsymia?
 I am so glad you asked! The CONQUER trial set out to answer that exact question.
I am so glad you asked! The CONQUER trial set out to answer that exact question.
What we know is there are a ton of neuronal and peripheral pathways involved in appetite regulation and obviously everyone is going to have varying degrees of regulation sensitivities, etc., hence why Obesity is considered a chronic disease.
Both Phentermine and Topiramate work along various pathways within this system, all of which haven’t been fully teased out, but they both seem to help by decreasing an individual’s appetite and reducing food-seeking behaviours.
Interesting! So what about that CONQUER trial?
 Yes, the CONQUER trial! What Gadde and friends did was run a double-blind, placebo-controlled trial.
Yes, the CONQUER trial! What Gadde and friends did was run a double-blind, placebo-controlled trial.
They had 3 groups and nearly 2500 participants.
- Group 1: Placebo
- Group 2 (Low-dose): Phentermine 7.5mg plus Topiramate 46mg once daily
- Group 3 (High-dose): Phentermine 15mg plus Topiramate 92mg once daily
All participants were monitored for a little over 1 year (56 weeks) and were also provided with some lifestyle counselling that included a LEARN manual (I am not actually sure what this means. I am assuming some kind of lifestyle modification workbook – I didn’t have enough time look it up; sue me), and they were instructed to reduce their calorie intake by 500 calories/day.
How did the trial go?
 One major negative of this study is that a huge number of people dropped out – 38% in total. This comprised of 43% in the placebo group, 31% in the low dose Qsymia group, and 36% in the high dose Qsymia group. As far as clinical trials go, this is a pretty appalling number.
One major negative of this study is that a huge number of people dropped out – 38% in total. This comprised of 43% in the placebo group, 31% in the low dose Qsymia group, and 36% in the high dose Qsymia group. As far as clinical trials go, this is a pretty appalling number.
Generally, if a study has > 20% drop-out we promptly shred it or perhaps use it as a fire starter. The reason being is that a drop-out rate of this level indicates some kind of flaw within the study design or an intolerability to the agent being tested. With a high number lost from the placebo group I suspect it was largely due to people not seeing the results they wanted or noting no benefit in terms of appetite reduction thereby informing them they were likely getting the placebo. This is of course all speculation.
The authors did gain some points by acknowledging the above; they also completed an Intention To Treat analysis (ITT). What an ITT means is they still include all the individuals at the beginning of the study that may have had only one dose of the drug and only completed the baseline assessment, and carried their data forward unchanged as if they had completed the study anyways.
So no good results worth sharing then?
Gadde and friends got some pretty solid results. As you can see from the graph below we have the completers, the people who stayed within their study group and participated in the study from start to finish. Then we have the ITT data. Obviously, the ITT data does not look as impressive, however, it provides a more realistic perspective of how the drug will do in real-life conditions!
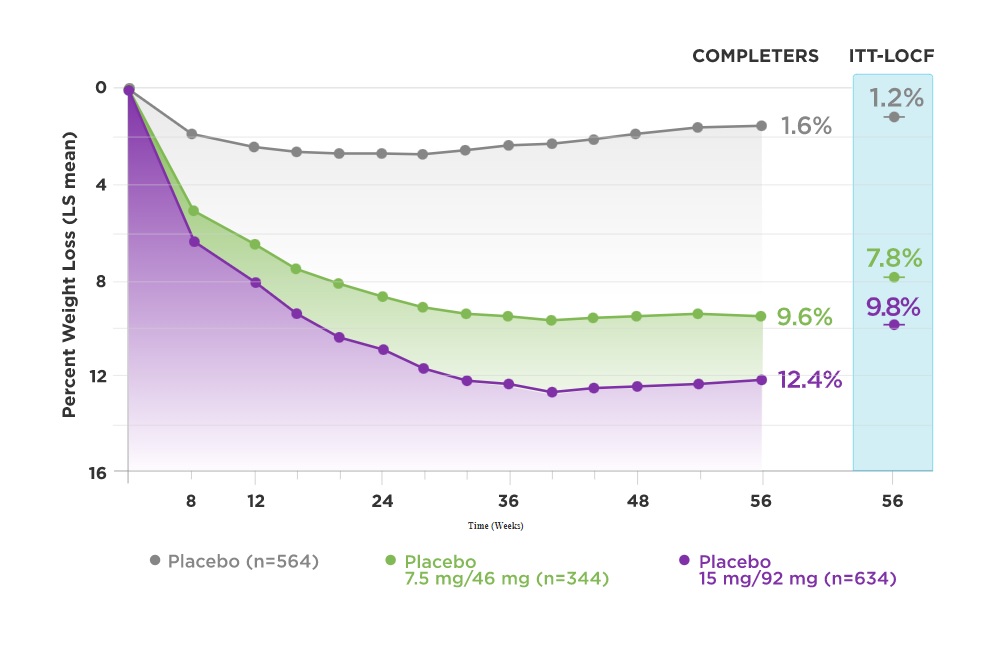
So, in conclusion, you can see that Qsymia 15/92mg daily may lead to a near 10% weight-loss from baseline and Qsymia 7.5mg/46mg may lead to a weight-loss of almost 8%, which really isn’t too shabby. The authors also found improvements in other markers and measures such as blood pressure and blood sugars. Overall, the medications were reasonably tolerated with a prickling sensation in the hands and feet being the most common side effect next to dizziness and headaches.
Will we ever see this medication become more available in other countries?
I am doubtful. I couldn’t find a specific reason for it being denied by Health Canada but I suspect it is due to Phentermine’s history with Fen-Phen. Trying to mitigate those future lawsuits and such, you know what I am saying?
That is all for today, my friends!
Always remember that small tweaks lead to massive peaks.